By: Nr. Chinonso Cynthia Ukah. BNSc, RN, RM. Freelance Health Writer and Datelinehealth Africa volunteer. Medically reviewed by: Dr. K. Craig, MB.BS; MPH:
A black lady testing blood glucose with a CGM system
• Continuous glucose monitoring systems (CGMs) are devices that track glucose levels 24 hours a day.
• CGMs consist of three parts: sensor, transmitter, and receiver.
• CGMs are currently too expensive for widespread use in the healthcare economy of Africa.
• Only four brands of continuous glucose monitors have been identified in Africa, used primarily for research purposes.
The African continent faces a significant diabetes burden, with data from the International Diabetes Federation suggesting that 55 million Africans could have diabetes by 2045. More concerning is that nearly half of adults with diabetes in Africa are unaware of their condition [1]. This growing epidemic calls for innovative approaches to diabetes management and care.
Continuous glucose monitoring (CGM) is one of those advanced approaches that track blood sugar levels in people with diabetes [2]. Unlike traditional finger-prick tests, CGM devices measure glucose in the fluid between body cells every few minutes. These devices are often a key component of artificial pancreas systems, although they are very different technologies [3].
This article provides an overview of CGM systems, their use, benefits, limitations, and challenges of implementing the technology across the continent.
Continuous glucose monitoring systems are devices that measure blood glucose levels 24/7, without the need for frequent finger pricks. They help people with diabetes maintain healthy glucose levels and reduce risks of both high and low blood sugar. This technology can be especially beneficial for diabetics who are likely to experience severe low blood sugar episodes.
CGM devices are typically used by patients at home. They are attached to the upper part of the arm or belly and consist of three components:
• A sensor
• A transmitter
• A receiver/smartphone [4]
The sensor is placed under the skin where it measures glucose in the interstitial fluid (the fluid between cells) every few minutes. It then transmits the glucose level results to the receiver. The receiver could be a smartphone or another monitor where the results can be read.
Some of these devices have an insulin pump built into them that delivers insulin once it detects a low blood sugar level. [5] An example is shown below in fig 1.
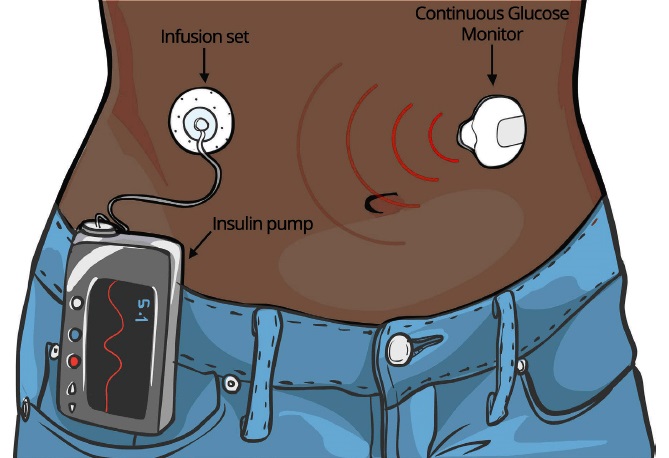
Fig. 1: Cartoon illustration of a black lady wearing a Continuous Glucose Monitoring System with an insulin pump.
Click on image to enlarge.
• People with type 1 diabetes
• Some people with type 2 diabetes
• Pregnant women with diabetes
• Specific high-risk groups in African populations
The use of Continuous Glucose Monitoring (CGM) systems is widespread in Western countries. In the United Kingdom, several brands are in use by patients with type 1 diabetes (see samples in fig. 2). [6].
Fig. 2: Samples of different CGM systems. Click on image to enlarge. Image credit: USMed
Limited use of CGM systems has been seen in studies in some African countries such as Uganda, Kenya, Malawi, and South Africa [7, 8]. They are, however, primarily used for research purposes due to barriers such as cost, which was repeatedly cited by researchers. Some CGM systems used in African studies include:
• FreeStyle Libre Pro
• FreeStyle Libre 2
• Dexcom G6
• Flash CGM
The FreeStyle Libre Pro was donated for a research study in Uganda and was well-accepted by the participants. This professional system is blinded, meaning the wearer can't see the results, which were only accessible to researchers [4].
The Flash CGM was studied among youths in urban East Africa, specifically in Malawi [7]. Hopes are high for its future clinical use in Africa if its price decreases.
1. Clean hands thoroughly with soap and water.
2. Select a site on the body, usually the upper arm or belly, avoiding areas with scars and tattoos.
3. Clean the chosen area with an alcohol swab and allow it to dry.
4. Prepare the sensor applicator according to the device manual.
5. Place the applicator on the skin and press to insert the sensor.
6. Connect the transmitter to the sensor if it's not already attached.
7. Dispose of the needle part safely in a designated container out of reach [8].
1. Keep the sensor area clean and dry.
2. Avoid applying creams or oils near the sensor site.
3. Inspect the sensor site daily to ensure it's in good condition.
4. Calibrate the CGM when prompted by the device [3].
5. Replace the sensor every 7-14 days, depending on the specific model.
6. Replace the transmitter battery as needed.
CGM devices monitor blood glucose levels throughout the day and night. They help keep blood sugar levels within a target “time in range,” typically 70-180 mg/dL. These devices help detect hypoglycemia, especially for pregnant women with diabetes who may have low blood sugar at night. Some versions can sound alarms when sugar levels are too high or too low [9].
People with diabetes in remote areas of Africa can potentially receive better care without traveling far. CGM data can be shared with doctors, who can analyze the results and provide advice remotely. They may suggest adjusting insulin doses or changing diet based on the CGM data [6].
CGM systems reduce the need for frequent finger-prick tests, which can lower the ongoing cost of test strips. By improving glucose control, CGMs may help reduce diabetes complications over time. They can also minimize the need for hospital visits, potentially lowering overall healthcare costs for people with diabetes.
Using CGM systems requires extensive training. Users need to learn how to set up the device, input information, change batteries, and troubleshoot problems [11]. These collectively constitute overwhelming "user burden", especially for older patients and those not comfortable with technology.
In the United States for example, the adoption rate of CGM system for all types of diabetes is reported to be as low as 8 - 17% due to the high perception of "user burden". In remote parts of Africa with low literacy for medical technologies generally and few diabetes specialists, getting proper training and support can be challenging and will demotivate users from adoption of the technology.
Like any technology, CGM systems can malfunction due to software glitches, hardware issues, or battery failures. If the device stops working suddenly, especially at night or away from home, it could be dangerous. Users always need a backup plan, such as carrying extra insulin and traditional testing supplies. This can add stress to daily life.
Most current systems still require users to input information, especially about meals. The device needs to be informed about food intake to calculate the right insulin dose. This means users still need to be actively involved in managing their diabetes, which can be difficult for some people, especially those who struggle with counting carbohydrates or have busy lifestyles.
CGM devices are prohibitively expensive in many African countries, with costs ranging from several hundred to a few thousand US dollars (in local currenciea) for the initial setup, plus ongoing costs for sensors and supplies. Prices may vary significantly between countries and specific systems. The sensors need replacing every 7-14 days, which adds to the ongoing costs. [10] For many Africans with diabetes, even regular glucose strips and food are a luxury, making these advanced devices out of reach.
Many African countries lack healthcare professionals trained in CGM technology. While few patients may acquire the device, they would still struggle to find doctors or nurses who can interpret the data and provide guidance. Without proper support, the benefits of CGM devices may not be fully realized, and patients might misinterpret readings or miss important trends in their glucose levels.
CGM devices require regular charging, much like smartphones. Frequent power outages can disrupt continuous monitoring, leading to gaps in data and potentially risky situations for patients relying on CGM alerts. Limited internet connectivity in rural areas can also hinder the real-time data sharing capabilities of some CGM systems.
In some African communities, wearing a medical device constantly might be viewed with skepticism. CGMs might be seen as a sign of severe illness, which can potentially lead to stigma. In neighborly communities, the constant health monitoring might raise privacy concerns.
• In areas with unreliable cellular networks, consider CGM systems that can store data locally for extended periods.
• During dry seasons; be aware that increased skin dryness might affect sensor adhesion.
• In regions with limited access to healthcare, encourage connection with diabetes support groups (online or in-person) for peer support and troubleshooting tips.
• Consult with a doctor or diabetes educator for guidance on proper CGM use.
CGM devices are not widely available across Africa. Their use is primarily limited to research studies in countries like Uganda, Kenya, Malawi, and South Africa.
While CGM devices provide more continuous data, they don't entirely replace traditional glucose meters. Many CGM systems still require occasional finger-prick tests for calibration or confirmation of readings [6].
Coverage varies greatly depending on the country and insurance provider. In many African countries, CGM devices are not covered by national health insurance schemes due to their high cost.
Yes, but it may require additional planning. Users might need to keep backup power banks charged for times when electricity is unavailable.
This depends on the specific CGM system, but most sensors need to be replaced every 7-14 days.
Most modern CGM devices are water-resistant and can be worn while swimming or exercising. However, it's important to check the specific guidelines for each device.
CGM devices measure glucose levels continuously, reducing the need for frequent finger-prick tests. Making these devices accessible and practical for widespread use in Africa will require addressing significant economic, infrastructural, and cultural challenges. As research progresses and technology advances, it's important for African healthcare systems to prepare for the potential integration of CGM devices into diabetes care strategies.
1. International Diabetes Federation. IDF Diabetes Atlas, Diabetes around the world 2021. 10th edition. Brussels, Belgium. [Internet, n.d.]. Cited July 14, 2024. Available from here.
2. Moser O, Riddell MC, Eckstein ML, Adolfsson P, Rabasa-Lhoret R, van den Boom L, Gillard P, Nørgaard K, Oliver NS, Zaharieva DP, Battelino T, de Beaufort C, Bergenstal RM, Buckingham B, Cengiz E, Deeb A, Heise T, Heller S, Kowalski AJ, Leelarathna L, Mathieu C, Stettler C, Tauschmann M, Thabit H, Wilmot EG, Sourij H, Smart CE, Jacobs PG, Bracken RM, Mader JK. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Pediatr Diabetes. 2020 Dec;21(8):1375-1393. doi: 10.1111/pedi.13105. Available from here.
3. Adler AJ, Ruderman T, Valeta F, et al. Protocol for a feasibility randomised control trial for continuous glucose monitoring in patients with type 1 diabetes at first-level hospitals in rural Malawi. BMJ Open. 2022;12(2):e052134. Available from here.
4. Alsaleh FM, Smith FJ, Keady S, Taylor KMG. Insulin pumps: from inception to the present and toward the future. J Clin Pharm Ther. 2010;35(2):127-138. Available from here.
5. Dowling L, Wilmot EG, Choudhary P. Do-it-yourself closed-loop systems for people living with type 1 diabetes. Diabetic Med. 2020;37(12):1977-1980. Available from here.
6. Diabetes UK. Closed loop systems (artificial pancreas) [Internet], n.d]. Cited 2023 Jul 14]. Available from here.
7. McClure Yauch L, Velazquez E, Piloya?Were T, et al. Continuous glucose monitoring assessment of metabolic control in East African children and young adults with type 1 diabetes: a pilot and feasibility study. Endocrinol Diabetes Metab. 2020;3(3):e00135. Available from here.
8. Distiller LA, Cranston I, Mazze R. First clinical experience with retrospective flash glucose monitoring (FGM) analysis in South Africa: characterizing glycemic control with ambulatory glucose profile. J Diabetes Sci Technol. 2016;10(6):1294-302. Available from here.
9. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. J Lab Med. 2020;44(2):71-9. Available from here.
10. Buschur EO, Faulds E, Dungan K. CGM in the hospital: is it ready for prime time?. Curr Diabetes Rep. 2022;22(9):451-60. Available from here.
11. Lee I, Probst D, Klonoff D, Sode K. Continuous glucose monitoring systems-Current status and future perspectives of the flagship technologies in biosensor research. Biosens Bioelectron. 2021;181:113054. Available from here.
Management of type 2 diabetes in Nigeria
Published: June 22, 2024
© 2024. Datelinehealth Africa Inc. All rights reserved.
Permission is given to copy, use and share content for non-commercial purposes without alteration or modification and subject to source attribution.
DATELINEHEALTH AFRICA INC., is a digital publisher for informational and educational purposes and does not offer personal medical care and advice. If you have a medical problem needing routine or emergency attention, call your doctor or local emergency services immediately, or visit the nearest emergency room or the nearest hospital. You should consult your professional healthcare provider before starting any nutrition, diet, exercise, fitness, medical or wellness program mentioned or referenced in the DatelinehealthAfrica website. Click here for more disclaimer notice.